At the isoelectric ph of a tetrapeptide – The isoelectric point (pI) of a tetrapeptide, a molecule composed of four amino acids, plays a crucial role in determining its behavior and properties. This article delves into the concept of pI, exploring the factors that influence it, methods for its determination, and its applications in protein science.
Understanding the pI of a tetrapeptide is essential for protein purification, characterization, and predicting its solubility and behavior in various environments. This comprehensive guide provides a thorough examination of this important aspect of peptide chemistry.
Isoelectric Point of a Tetrapeptide

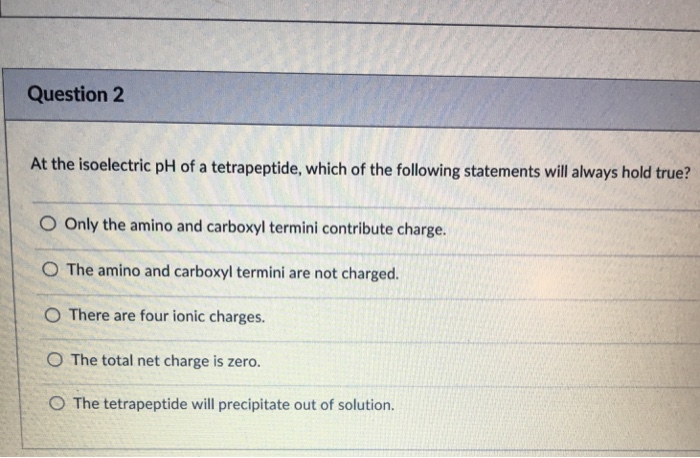
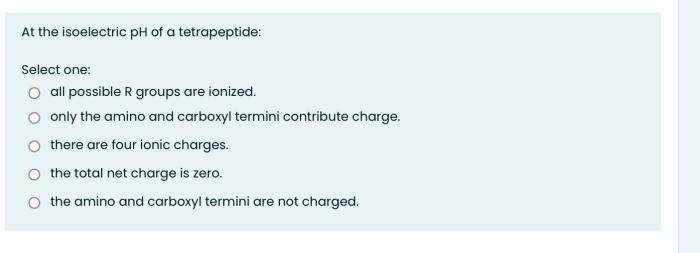
The isoelectric point (pI) of a peptide is the pH at which the net charge of the peptide is zero. At this pH, the peptide has an equal number of positive and negative charges and is therefore electrically neutral. The pI of a tetrapeptide is determined by the amino acid composition of the peptide.
Amino acids with acidic side chains (such as glutamic acid and aspartic acid) contribute negative charges to the peptide, while amino acids with basic side chains (such as lysine and arginine) contribute positive charges. The pI of a tetrapeptide is the pH at which the sum of the negative charges and the sum of the positive charges are equal.
Factors Affecting Isoelectric Point
The amino acid composition of a tetrapeptide is the primary factor that determines its pI. The more acidic amino acids a tetrapeptide contains, the lower its pI will be. Conversely, the more basic amino acids a tetrapeptide contains, the higher its pI will be.
The pH of the solution also affects the charge of amino acids and peptides. At low pH, amino acids are protonated and have a positive charge. At high pH, amino acids are deprotonated and have a negative charge. The pH of the solution can therefore be used to control the charge of a tetrapeptide and to determine its pI.
Methods for Determining Isoelectric Point, At the isoelectric ph of a tetrapeptide
There are several experimental methods that can be used to determine the pI of a tetrapeptide. One common method is isoelectric focusing. In isoelectric focusing, a tetrapeptide is subjected to an electric field in a pH gradient. The tetrapeptide will migrate to the pH at which its net charge is zero, which is its pI.
Another common method for determining the pI of a tetrapeptide is capillary electrophoresis. In capillary electrophoresis, a tetrapeptide is subjected to an electric field in a capillary tube. The tetrapeptide will migrate to the pH at which its net charge is zero, which is its pI.
Applications of Isoelectric Point
The pI of a tetrapeptide is an important property that can be used for protein purification and characterization. By knowing the pI of a tetrapeptide, it is possible to design purification methods that will selectively isolate the tetrapeptide from a mixture of other proteins.
The pI of a tetrapeptide can also be used to predict its solubility and behavior. For example, tetrapeptides with low pIs are more soluble in acidic solutions, while tetrapeptides with high pIs are more soluble in basic solutions.
Answers to Common Questions: At The Isoelectric Ph Of A Tetrapeptide
What is the isoelectric point of a peptide?
The isoelectric point (pI) of a peptide is the pH at which the net charge of the peptide is zero. At this pH, the peptide has an equal number of positively and negatively charged groups.
How is the pI of a tetrapeptide determined?
The pI of a tetrapeptide can be determined experimentally using methods such as isoelectric focusing or capillary electrophoresis. These methods separate peptides based on their charge at different pH values, allowing for the identification of the pI.