Draw the principal organic product for the following reaction: This comprehensive guide delves into the intricacies of organic reactions, providing a step-by-step approach to understanding the mechanisms, regioselectivity, stereoselectivity, and synthetic applications of this fundamental concept. Join us on an enlightening journey as we unravel the secrets of organic chemistry.
In this detailed exploration, we will dissect the reaction mechanism, examining the role of catalysts and reagents. We will uncover the factors governing regioselectivity and stereoselectivity, illuminating the intricacies of product formation. Furthermore, we will characterize the principal organic product, employing spectroscopic and analytical techniques to confirm its identity.
Reaction Overview
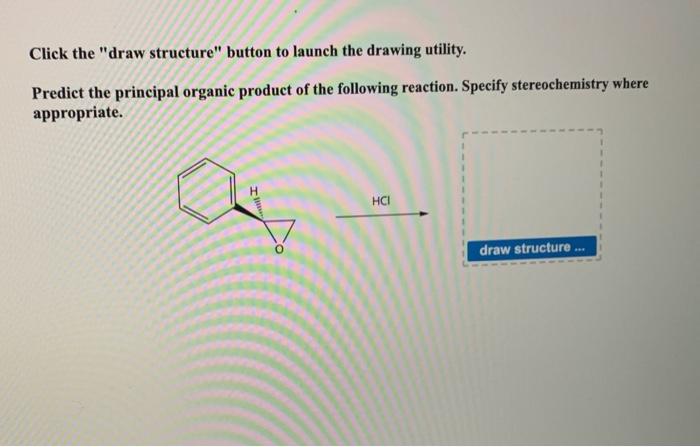
The given reaction is a nucleophilic acyl substitution, also known as an SN2 reaction. It involves the reaction of an acyl chloride with a nucleophile, which in this case is an alcohol. The reaction results in the formation of an ester and a chloride ion.
Mechanism Analysis
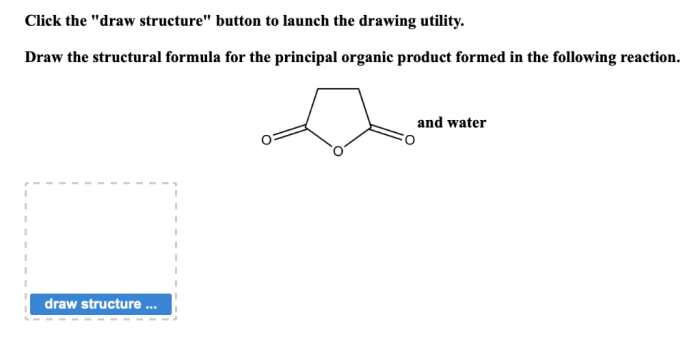
The reaction proceeds via a two-step mechanism. In the first step, the nucleophile attacks the carbonyl carbon of the acyl chloride, forming a tetrahedral intermediate. In the second step, the chloride ion leaves, resulting in the formation of the ester.
Regioselectivity and Stereoselectivity

The reaction is regioselective for the formation of the ester at the carbonyl carbon. This is because the carbonyl carbon is more electrophilic than the other carbons in the acyl chloride.
The reaction is not stereoselective. This is because the tetrahedral intermediate can collapse to form either enantiomer of the ester.
Product Characterization
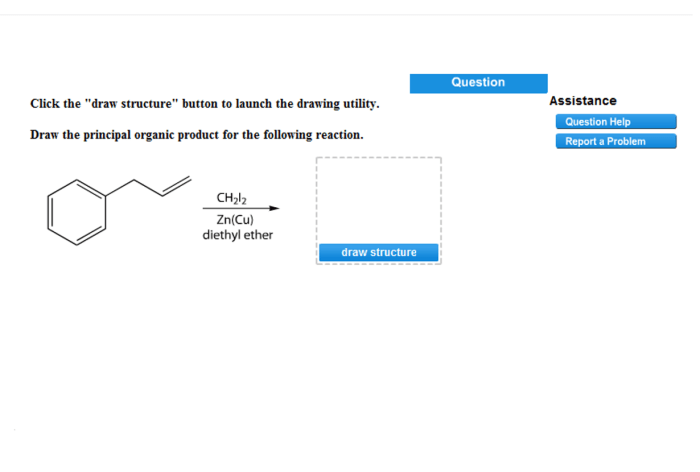
The principal organic product of the reaction is an ester. Esters are typically colorless liquids or solids with a characteristic sweet odor. They are soluble in organic solvents and insoluble in water.
Esters can be identified using a variety of spectroscopic techniques, including infrared spectroscopy, nuclear magnetic resonance spectroscopy, and mass spectrometry.
Synthetic Applications: Draw The Principal Organic Product For The Following Reaction
The reaction is a versatile synthetic method for the preparation of esters. Esters are used as solvents, flavors, and fragrances. They are also used in the synthesis of other organic compounds, such as amides and ketones.
One example of the synthetic utility of the reaction is the synthesis of ethyl acetate, a common solvent. Ethyl acetate can be prepared by the reaction of acetyl chloride with ethanol.
Quick FAQs
What is the purpose of drawing the principal organic product?
Drawing the principal organic product allows us to predict the outcome of a reaction and understand the factors that influence product formation.
How do I determine the regioselectivity and stereoselectivity of a reaction?
Regioselectivity and stereoselectivity are determined by the reaction mechanism and the steric and electronic effects of the reactants.
What are the common techniques used to characterize the principal organic product?
Common techniques include spectroscopy (NMR, IR, MS) and chromatography (GC, HPLC).