The mole and Avogadro’s number worksheet provides a comprehensive exploration of these fundamental concepts in chemistry, guiding students through the intricacies of quantitative analysis and the precise measurement of chemical substances.
This worksheet delves into the concept of the mole as a unit of measurement, examining its relationship to atomic mass and its pivotal role in stoichiometric calculations. Furthermore, it explores Avogadro’s number, its significance in chemistry, and its historical determination.
The Mole Concept

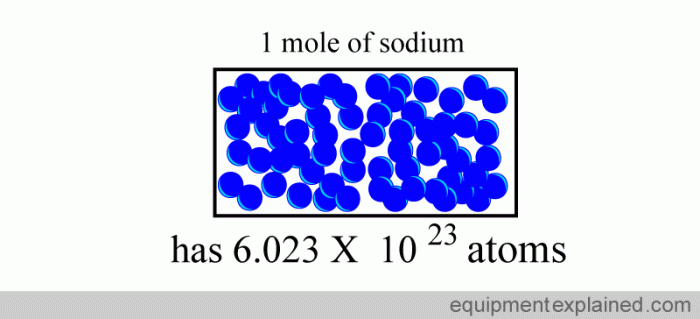
The mole is a unit of measurement for chemical substances. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12. The mole is a very large unit, so it is often convenient to use smaller units such as the millimole (mmol) or the micromole (µmol).
The mole is related to the atomic mass of an element. The atomic mass of an element is the average mass of the atoms of that element, weighted according to their relative abundance. The atomic mass of an element is expressed in grams per mole.
The mole is used in stoichiometric calculations to determine the amount of reactants and products in a chemical reaction. Stoichiometry is the study of the quantitative relationships between the reactants and products in a chemical reaction. Stoichiometric calculations are used to predict the amount of product that will be formed in a reaction or to determine the amount of reactant that is needed to produce a given amount of product.
Examples of the Mole Concept
- The molar mass of sodium chloride (NaCl) is 58.44 g/mol. This means that 58.44 g of NaCl contains 6.022 × 10 23formula units of NaCl.
- The balanced chemical equation for the reaction between hydrogen and oxygen is 2H 2+ O 2→ 2H 2O. This equation tells us that 2 moles of hydrogen react with 1 mole of oxygen to produce 2 moles of water.
Avogadro’s Number: The Mole And Avogadro’s Number Worksheet
Avogadro’s number is the number of atoms in 0.012 kilograms of carbon-12. It is equal to 6.022 × 10 23. Avogadro’s number is a very large number, so it is often convenient to use smaller units such as the millimole (mmol) or the micromole (µmol).
Avogadro’s number was first determined by the Italian scientist Amedeo Avogadro in 1811. Avogadro proposed that equal volumes of gases at the same temperature and pressure contain the same number of molecules. This hypothesis was later confirmed by the work of other scientists, and it is now known as Avogadro’s law.
Avogadro’s number has a wide range of applications in chemistry. It is used to determine the molar mass of a substance, to calculate the number of atoms in a sample of a substance, and to convert between the number of atoms and the number of moles.
Applications of Avogadro’s Number
- To determine the molar mass of a substance, divide the mass of the substance by the number of moles of the substance.
- To calculate the number of atoms in a sample of a substance, multiply the number of moles of the substance by Avogadro’s number.
- To convert between the number of atoms and the number of moles, divide the number of atoms by Avogadro’s number.
Worksheet Activities
Activity 1: Calculating the Number of Moles in a Given Mass of a Substance
Calculate the number of moles in 10.0 g of sodium chloride (NaCl).
Solution:
- Find the molar mass of NaCl: 58.44 g/mol
- Convert the mass of NaCl to grams: 10.0 g
- Divide the mass of NaCl by the molar mass of NaCl: 10.0 g / 58.44 g/mol = 0.171 mol
Therefore, there are 0.171 moles of NaCl in 10.0 g of NaCl.
Activity 2: Using Avogadro’s Number to Convert Between the Number of Atoms and the Number of Moles
Convert 1.00 × 10 23atoms of carbon to moles of carbon.
Solution:
- Divide the number of atoms of carbon by Avogadro’s number: 1.00 × 1023atoms / 6.022 × 10 23atoms/mol = 0.166 mol
Therefore, 1.00 × 10 23atoms of carbon is equal to 0.166 mol of carbon.
Activity 3: Applying the Mole Concept and Avogadro’s Number to Solve Real-World Problems
A chemist needs to prepare 100.0 mL of a 0.100 M solution of sodium chloride (NaCl). How many grams of NaCl does the chemist need to weigh out?
Solution:
- Calculate the number of moles of NaCl needed: 0.100 M × 0.100 L = 0.0100 mol
- Convert the number of moles of NaCl to grams: 0.0100 mol × 58.44 g/mol = 0.584 g
Therefore, the chemist needs to weigh out 0.584 g of NaCl to prepare 100.0 mL of a 0.100 M solution of NaCl.
Additional Resources

Online Resources, The mole and avogadro’s number worksheet
Books and Articles
- Chemistry: The Central Scienceby Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E. Bursten, and Catherine J.
Murphy
- General Chemistryby Raymond Chang and Kenneth A. Goldsby
Videos and Simulations
Common Queries
What is the mole concept?
The mole is a unit of measurement that represents a specific quantity of a substance, defined as the amount containing exactly 6.022 × 10^23 entities (atoms, molecules, ions, or electrons).
What is Avogadro’s number?
Avogadro’s number is a numerical constant, approximately 6.022 × 10^23, which represents the number of entities present in one mole of a substance.
How are the mole concept and Avogadro’s number related?
The mole concept and Avogadro’s number are closely related, as the mole is defined based on Avogadro’s number. One mole of any substance contains exactly Avogadro’s number of entities.