How many grams are in 88.1 moles of mg – Embarking on a scientific exploration, we delve into the enigmatic realm of chemistry to unravel the mysteries of how many grams reside within 88.1 moles of magnesium (Mg). This intriguing inquiry invites us to traverse the intricate relationship between mass and quantity, unveiling the secrets of molecular composition and the fascinating properties of this essential element.
As we navigate the intricacies of this conversion, we will decipher the fundamental concepts of grams and moles, unraveling the formula that seamlessly transforms one unit into the other. Through illustrative examples, we will witness the practical application of these principles, solidifying our understanding of the quantitative nature of chemical substances.
Grams and Moles
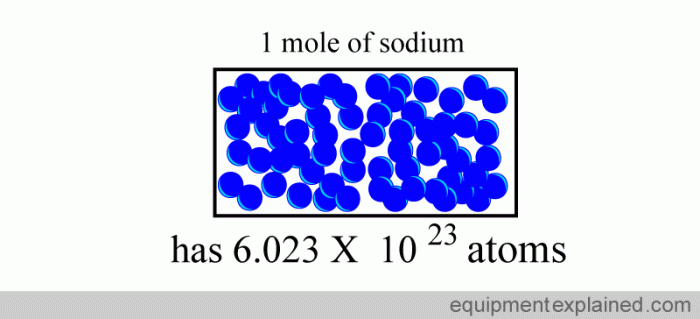
Grams and moles are two units used to measure the amount of a substance. A mole is defined as the amount of substance that contains exactly 6.02214076 × 10 23elementary entities. These entities can be atoms, molecules, ions, or electrons.
A gram is a unit of mass equal to one-thousandth of a kilogram.
Calculating Grams from Moles: How Many Grams Are In 88.1 Moles Of Mg

To calculate the number of grams in a given number of moles, we can use the following formula:
grams = moles × molar mass
where molar mass is the mass of one mole of the substance.
Molar Mass
Molar mass is the mass of one mole of a substance. It is calculated by adding the atomic masses of all the atoms in the molecule. For example, the molar mass of magnesium (Mg) is 24.305 g/mol.
Magnesium (Mg)

Magnesium is a lightweight, silvery-white metal. It is the eighth most abundant element in the Earth’s crust. Magnesium is used in a variety of applications, including alloys, batteries, and fertilizers.
Conversion

To convert 88.1 moles of Mg to grams, we can use the following formula:
grams = moles × molar mass
where the molar mass of Mg is 24.305 g/mol.
Plugging in the values, we get:
grams = 88.1 moles × 24.305 g/mol = 2143.6 g
Additional Considerations
When converting between grams and moles, it is important to be aware of the following considerations:
- Rounding errors: When converting between grams and moles, it is important to be aware of rounding errors. For example, if we round the molar mass of Mg to 24 g/mol, then the converted value would be 2112 g instead of 2143.6 g.
- Significant figures: It is also important to be aware of significant figures when converting between grams and moles. For example, if we only have two significant figures for the number of moles, then the converted value would be 2100 g instead of 2143.6 g.
FAQ Summary
What is the significance of molar mass in this conversion?
Molar mass serves as the bridge between the mass and quantity of a substance. It represents the mass of one mole of a substance, providing a crucial conversion factor that enables us to seamlessly transform moles into grams and vice versa.
How does the number of significant figures affect the accuracy of the conversion?
The number of significant figures in the given value of moles directly influences the precision of the converted mass. Adhering to the rules of significant figures ensures that the calculated mass retains the same level of accuracy as the original mole value, preventing the introduction of unwarranted precision.