How many atoms are in 4.39 g of co2 – Diving into the realm of chemistry, we embark on a journey to determine the number of atoms within 4.39 grams of carbon dioxide (CO2). This exploration unveils the fundamental concepts of molar mass, Avogadro’s number, and their profound implications in unraveling the microscopic world.
Carbon dioxide, a ubiquitous compound, plays a pivotal role in various natural processes and industrial applications. Understanding its atomic composition is crucial for deciphering its behavior and significance in the grand scheme of things.
Overview of Carbon Dioxide

Carbon dioxide (CO2) is a colorless, odorless, and non-flammable gas. It is composed of one carbon atom and two oxygen atoms, arranged in a linear molecular structure. Carbon dioxide is a naturally occurring gas that is released into the atmosphere through various processes, including respiration, decomposition, and volcanic eruptions.
CO2 is a greenhouse gas, meaning it traps heat in the atmosphere. This property contributes to the greenhouse effect, which is a major factor in global climate change. However, CO2 also plays an important role in the carbon cycle, which is essential for life on Earth.
Physical and Chemical Properties
Carbon dioxide is a relatively stable gas at room temperature and pressure. It is denser than air and has a slightly sour taste. CO2 is soluble in water, and it forms carbonic acid when dissolved. Carbon dioxide is also a non-flammable gas, making it useful as a fire suppressant.
Sources and Uses
Carbon dioxide is released into the atmosphere through both natural and human activities. Natural sources of CO2 include respiration, decomposition, and volcanic eruptions. Human activities that release CO2 include the burning of fossil fuels, deforestation, and industrial processes.
CO2 has a variety of uses, including:
- As a fire suppressant
- As a refrigerant
- As a carbonating agent in beverages
- As a fertilizer
- As a feedstock for the production of chemicals
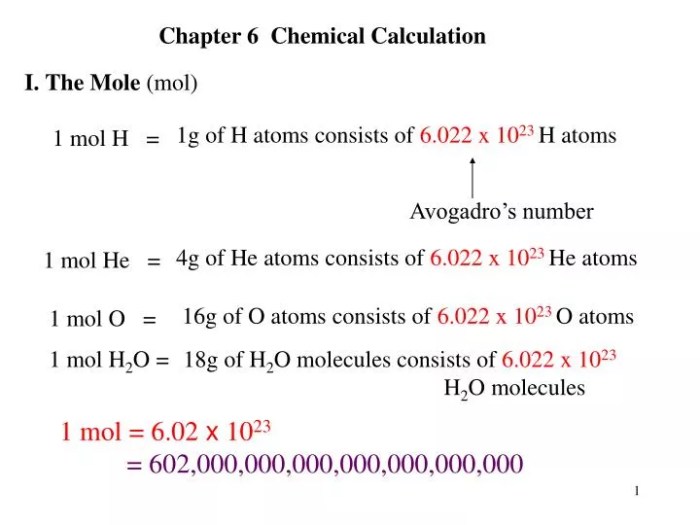
Molar Mass and Avogadro’s Number: How Many Atoms Are In 4.39 G Of Co2
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It represents the average mass of all the atoms or molecules in a compound.
Avogadro’s number is the number of atoms or molecules present in one mole of a substance. It is approximately 6.022 x 10 23and is used to convert between the mass and the number of atoms or molecules in a sample.
Formula for Calculating Number of Atoms
The number of atoms (N) in a given mass (m) of a substance can be calculated using the following formula:
N = (m / M) x NA
where:* m is the mass of the substance in grams
- M is the molar mass of the substance in grams per mole
- N Ais Avogadro’s number (6.022 x 10 23atoms/mol)
Calculating Atoms in CO2
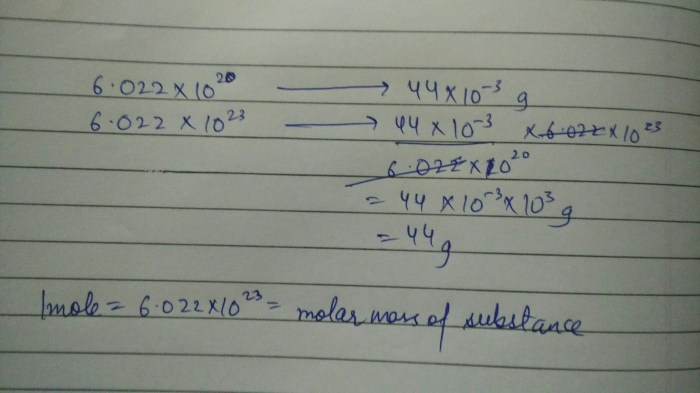
To determine the number of atoms present in 4.39 g of carbon dioxide (CO2), we need to follow a series of steps involving conversions, calculations, and the application of Avogadro’s number.
Converting Mass to Grams
The given mass of CO2 is 4.39 g, which is already in grams.
Determining Molar Mass of CO2
The molar mass of CO2 is the sum of the atomic masses of its constituent atoms: carbon (C) and oxygen (O). The atomic mass of carbon is 12.01 g/mol, and the atomic mass of oxygen is 16.00 g/mol. Therefore, the molar mass of CO2 is:
Molar mass of CO2 = (1 x 12.01 g/mol) + (2 x 16.00 g/mol) = 44.01 g/mol
Calculating Number of Moles of CO2, How many atoms are in 4.39 g of co2
The number of moles of CO2 present in 4.39 g can be calculated using the formula:
Number of moles = Mass / Molar mass
Substituting the given values:
Number of moles of CO2 = 4.39 g / 44.01 g/mol = 0.0997 moles
Using Avogadro’s Number to Calculate Number of Atoms
Avogadro’s number is a constant that represents the number of atoms, molecules, or ions present in one mole of a substance. It is equal to 6.022 x 10^23 particles per mole.
Using Avogadro’s number, we can calculate the number of atoms in 0.0997 moles of CO2:
Number of atoms = Number of moles x Avogadro’s number
Substituting the values:
Number of atoms = 0.0997 moles x 6.022 x 10^23 particles/mole = 6.00 x 10^21 atoms
Therefore, there are approximately 6.00 x 10^21 atoms present in 4.39 g of carbon dioxide (CO2).
Understanding the Results
The calculated number of atoms provides valuable insights into the composition of 4.39 g of carbon dioxide (CO 2).
The relationship between mass, moles, and the number of atoms is fundamental to understanding the results. The mass of a substance represents the total amount of matter present, while the mole is a unit of measurement that represents a specific quantity of atoms or molecules.
Avogadro’s number, which is approximately 6.022 × 10 23atoms per mole, establishes the connection between the mole and the number of atoms.
Significance of the Calculated Number of Atoms
The calculated number of atoms indicates the exact number of carbon and oxygen atoms present in 4.39 g of CO 2. This information is crucial for understanding the stoichiometry of chemical reactions involving CO 2and for determining the precise composition of mixtures containing CO 2.
Essential FAQs
What is the molar mass of carbon dioxide?
The molar mass of carbon dioxide (CO2) is approximately 44.01 g/mol.
How many moles of CO2 are present in 4.39 g?
To determine the number of moles, we divide the mass (4.39 g) by the molar mass (44.01 g/mol), yielding approximately 0.1 mol of CO2.
How many atoms are in 0.1 mol of CO2?
Using Avogadro’s number (6.022 x 10^23 atoms/mol), we multiply the number of moles (0.1 mol) by Avogadro’s number, resulting in approximately 6.022 x 10^22 atoms in 4.39 g of CO2.